Lactobacillus Reuteri Prodentis, Probiotic for Maintenance of Healthy Gums
Periodontal disease is among the most common of human diseases, arising due to an imbalance between pathogenic bacteria and desirable or beneficial microorganisms. The susceptibility of the host also plays a significant role and can be influenced by a range of circumstances, dietary and lifestyle choices, or diseases.

Initial therapy for periodontal diseases focuses on reducing the number of unwanted pathogens through scaling and root planing (SRP). For these same patients, numerous studies confirm that the probiotic bacteria Lactobacillus reuteri Prodentis (LrP), represents a useful therapeutic supplement in anti-infective therapy, and for periodontal maintenance therapy post intervention.
Studies also show that some bacteria have both anti-microbial and anti-inflammatory properties. The aim of treatment with Lactobacillus reuteri Prodentis is to sustainably increase the proportion of beneficial bacteria in the oral cavity and to restore the natural balance of the oral flora.
Antimicrobial, anti-inflammatory, and plaque inhibitory effects of Lactobacillus reuteri Prodentis are confirmed in multiple studies.
In the study by Teughels et al. 30 chronic periodontitis patients were recruited and monitored clinically and microbiologically at baseline, 3, 6, 9 and 12 weeks after therapy. All patients received one-stage full-mouth disinfection and were randomly assigned over a test (SRP + probiotic, n = 15) or control (SRP + placebo, n = 15) group. The lozenges were used two times a day for 12 weeks.
The result shows that the patient group receiving Lactobacillus reuteri Prodentis had significantly fewer patients with deep pockets. A significant reduction in the pocket depth of deep pockets as well as an attachment gain were also observed, and the pathogenic germ Porphyromonas gingivalis was significantly more contained (- 1.17 cfu/ml vs. - 0.22 cfu/ml in saliva after 12 weeks).
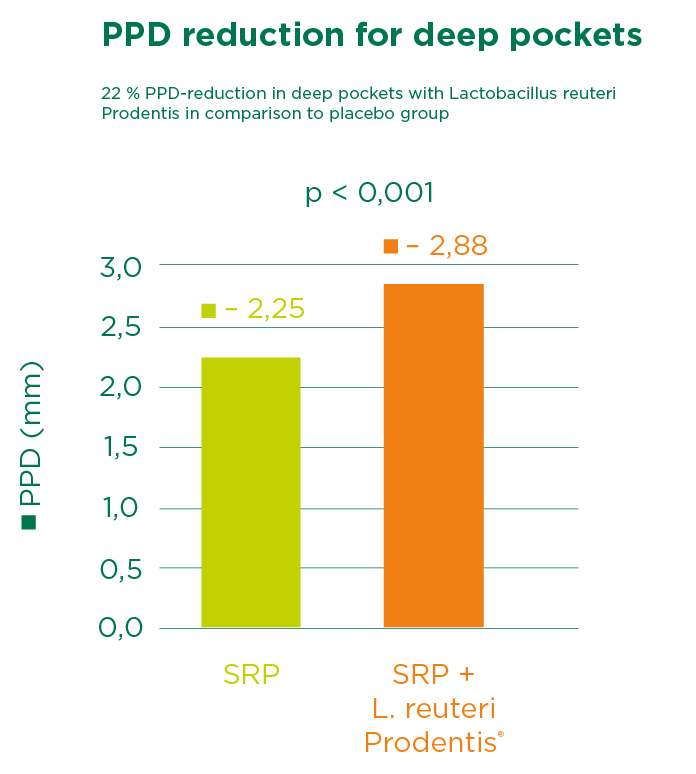
The double-blind study by Vivekananda et al. confirms these results. Half of the 30 periodontitis study participants were treated with SRP, while the other served as control.
From day 21 to 42, one group received two tablets of Lactobacillus reuteri Prodentis per day, and the other group a placebo. In all treated patients, the plaque index (PI), the gingiva index (GI) and the gingiva bleeding index (GBI) improved significantly, although to differing levels.
While the most significant effect was observed with combination of SRP and Lactobacillus reuteri Prodentis, the combination of SRP plus placebo actually produced worse results than treatment with Lactobacillus reuteri Prodentis alone.
In patients treated with SRP plus Lactobacillus reuteri Prodentis, pocket depth was reduced from 5,08 to 3,78 mm and the clinical attachment level was reduced from 3,93 to 2,85 mm.
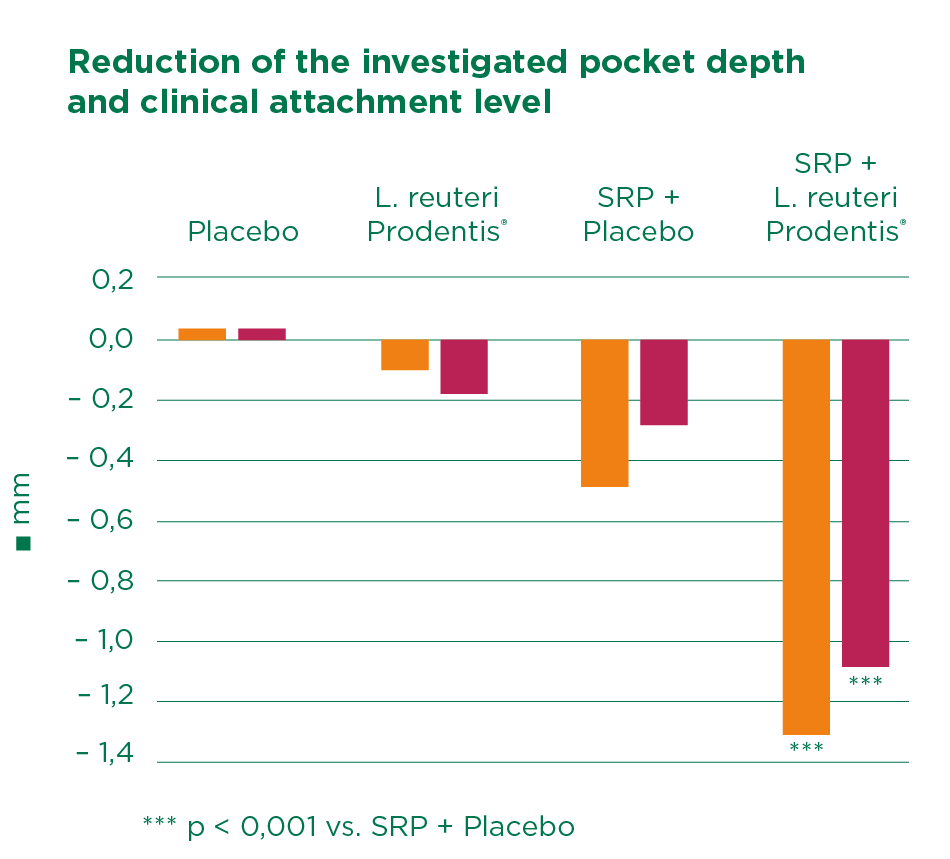
The study confirmed the plaque inhibition, anti-inflammatory, and antimicrobial effects of Lactobacillus reuteri Prodentis. The authors concluded that considering the beneficial effects of probiotics, this therapy could serve as a useful adjunct or alternative to periodontal treatment when SRP might be contraindicated.
Martin Cabezas and colleagues undertook a meta-analysis at the Osteoarticular & Dental Regenerative Nanomedicine unit at INSERN in Strasburg, France. The co-authors included Randomized controlled trials (RCTs) comparing SRP + probiotic versus SRP alone. This work confirmed the beneficial effects of probiotic treatment and supports the adjunctive use of Lactobacillus reuteri Prodentis to SRP in chronic periodontitis treatment, especially for patients with deep pockets.
Have a look at further study results from various risk groups: The influence of our diet on periodontitis, Probiotics reduce Candida albicans in elderly patients, Probiotics in pregnancy and Probiotics and their use in peri-implant mucositis.
[1] Teughels et al. (J Clin Periodontol) 2013, 1025–35.
[2] Vivekananda, et al. (J Oral Microbiology 2010, 2; 5344-ff).
[3] Rodrigo Martin-Cabezas, et al. (J of Clinical Periodontology 2016; 520-530).